Pharm Res –511, 05), these and other critical issues were addressed A practical, iterative, "fitforpurpose" approach to biomarker method development and validation is proposedPK Assays We offer assay development, validation, and implementation using ELISAs and MesoScale Discovery (MSD) assays for biologics;We can develop or transfer a method, optimize, qualify or validate customized assays in multiple application fields detection and quantification of soluble biomarkers, immunophenotyping, ELISpot, antidrug antibody detection, pharmacokinetic studies, etc Our assay validation process relies on bioanalytical method validation guidelines from
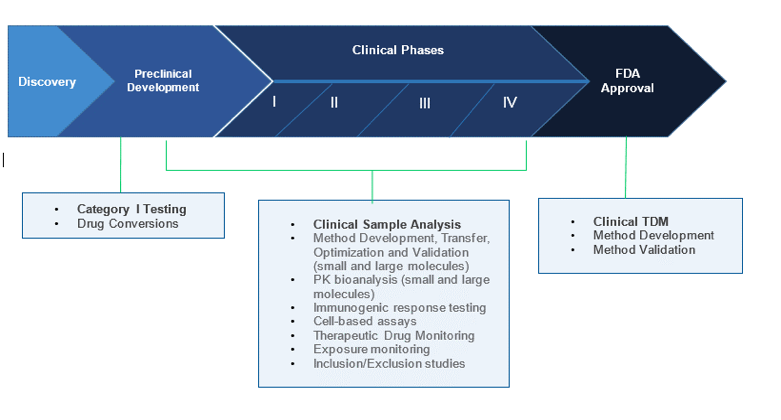
Research Phases Nms Labs
Pk assay development and validation
Pk assay development and validation-Active Drug Assays and Current Guidelines Challenges/Limitations 7 Active drug assays can be validated according to current PK assay validation guidelines (EMA, FDA, ) for some aspects However, the guidelines do not consider special needs of active drug assays for validation and sample analysis Ø General validation concept An assay's development phase requires continuous evaluation that should be clearly distinct from the validation phase The regulatory and statistical requirements for assay development and validation are different Understanding the differences between them is crucial for discussing the statistical considerations in each phase
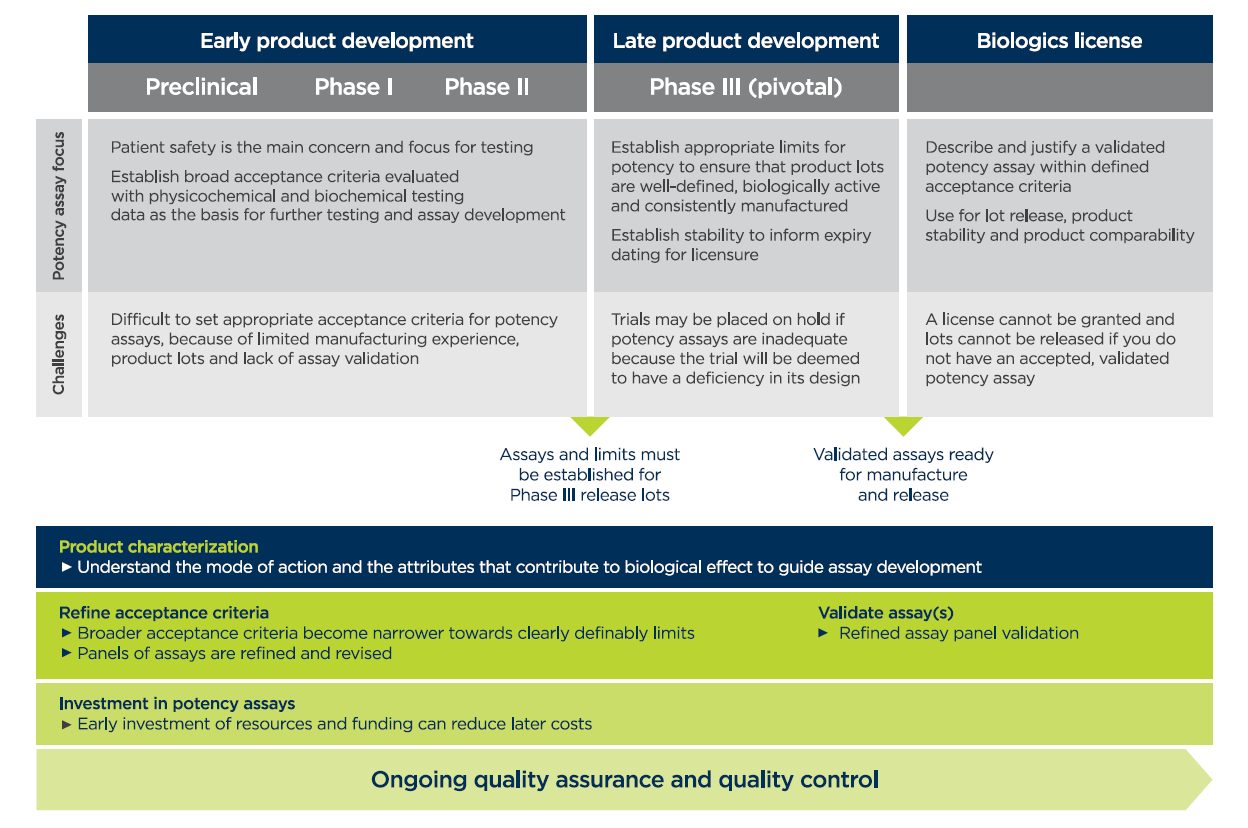



Potency Assays For Atmps Overcoming Challenges On The Path To Commercialization Insights From Our Labs To Yours
Upon completion of this course, attendees will have a clear understanding of regulatory agency expectations for bioanalytical development, and will have gained the background knowledge necessary to effectively plan bioanalytical assay development and validation programs for both quantitation assays and PK studiesValidation procedures though other ELISA detection methods such as horse radish peroxidase (HRP) reporting methods are equivalent The assays employ a sandwich immunoassay format where capture antibodies are coated on the bottom of the wells of a specialized ECL compatible 96well plate The sample and 5 Lee JW, Devanarayan V, Barrett YC et al Fitforpurpose method development and validation for successful biomarker measurement Pharm Res 23(2),312–328 (06) Pertains to regulatory or industry perspectives/positions on generally accepted practices for PK and biomarker assaysCrossref, Medline, CAS, Google Scholar
And Quanterix SIMOA® for ultrasensitive detectionMacokinetic (PK) assessments derived from serum drug concentration, receptor occupancy data can be used to model PK/PD relationships and validate dose selection decisions throughout the drug development lifecycle Receptor occupancy assays can be even more challenging to develop than other flow cytometric methods (eg surface immunophenotyping)The concept of ADCs is not new;
Cellbased Assays PPD ® Laboratories' bioanalytical lab has been providing cellbased assays services since 07 We have a dedicated team of research scientists experienced in cell culture technologies, cellbased assay development and validation, cellline engineering, microbiology, virology and molecular biologyQuantitative bioanalysis of antibodyconjugated payload in monkey plasma using a hybrid immunocapture LCMS/MS approach Assay development, validation, and a case study J Chromatogr B Analyt Technol Biomed Life SciFlow cytometry and Droplet Digital™ PCR (ddPCR) for cellbased therapies;



2
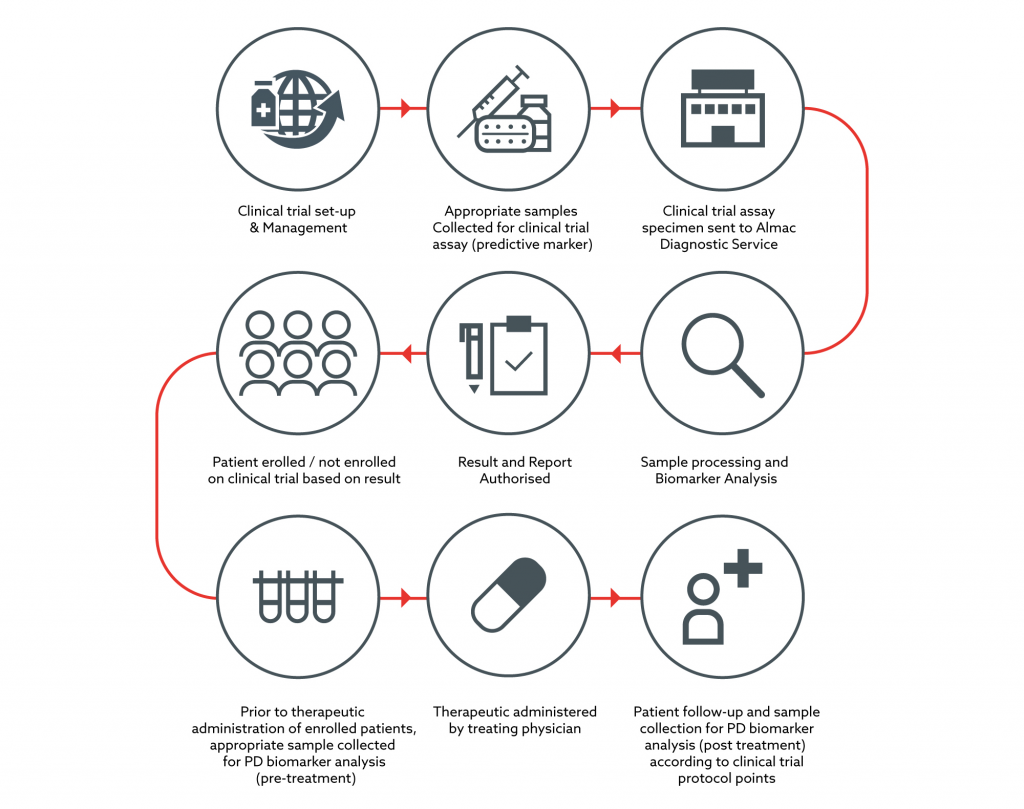



Pharmacodynamic Biomarker Testing Almac
Assay Development and Method Validation Essentials Thomas A Little PhD President Thomas A Little Consulting N Wildflower Lane Highland, UT drlittle@drtomcom Fundamental to all aspects of drug development and manufacturing are the analytical methodsOur scientists have longstanding experience in PK biomarker assessment including assay development and validation under GLP quality standards The result of PK biomarker assessment studies at Creative Biolabs is accurate and reliable to ensure highquality PK assay services for our clients all over the world As innovator data are readily available, the PK method development and validation should be targeted to the expected exposure data Such was the case of NESP and its biosimilar within our laboratory Multiple attempts of using monoclonal and polyclonal antibodies on the ELISA, MSD, and Gyros platforms resulted in a bioanalytical method with ng




Recommendations For Adaptation And Validation Of Commercial Kits For Biomarker Quantification In Drug Development Bioanalysis
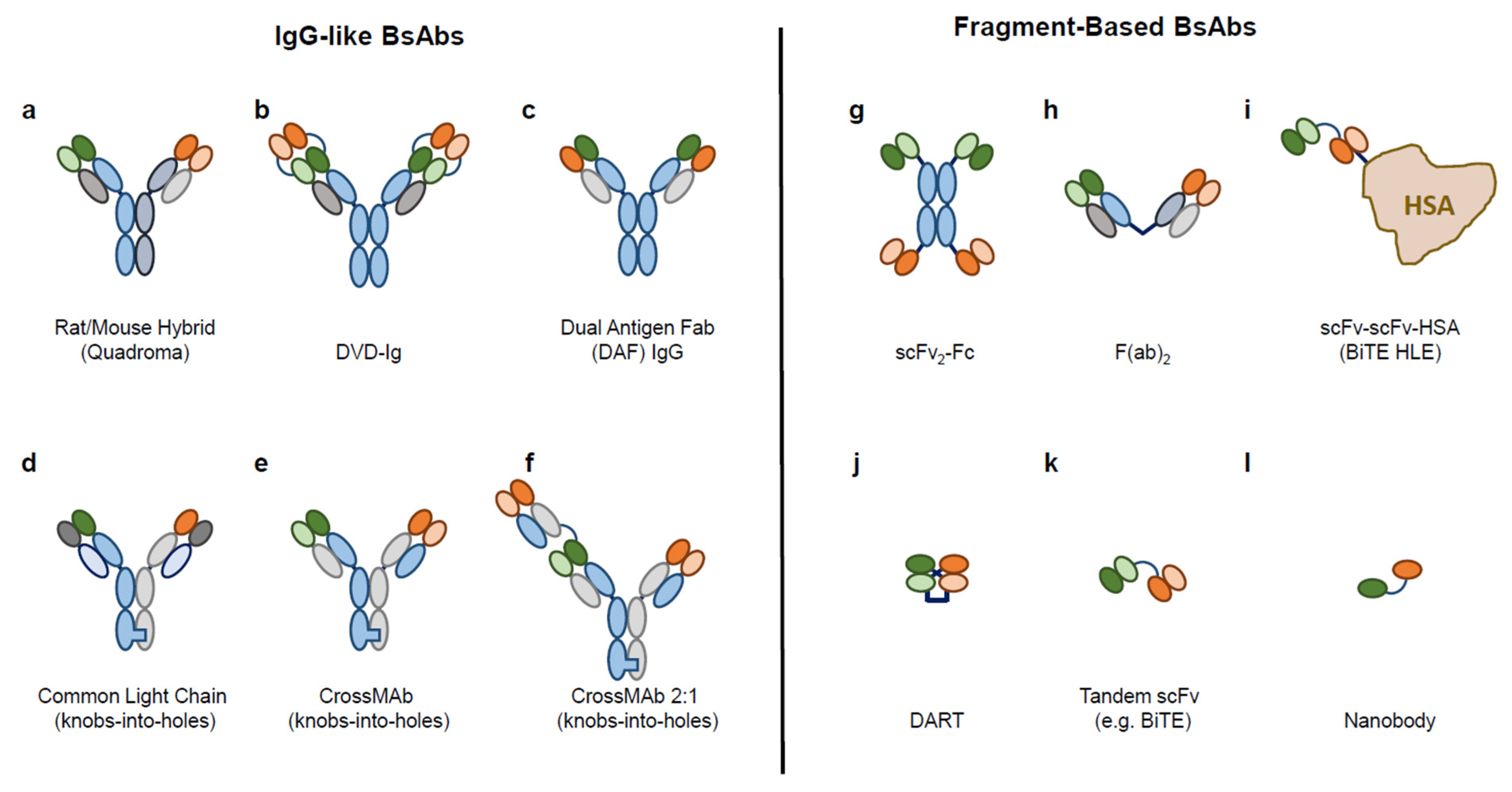



Ijms Free Full Text Bioassay Development For Bispecific Antibodies Challenges And Opportunities Html
(similar concept to process validation) Stage 1 – Assay Design The assay is defined during this stage based on knowledge gained through development activities Stage 2 – Assay Qualification During this stage, the assay design is confirmed as being capable of producing reproducible results suitable for the specified purpose Scientifically sound work inFlow cytometry and Droplet Digital™ PCR (ddPCR) for cellbased therapies;The development and validation of a PK assay can be challenging due to matrixinterferences and drugdrug interactions Interfering matrix components such as lipids, serum proteins, anticoagulants, antidrug antibodies or other factors such as ionic strength, pH or viscosity or other administered drugs, might result in unreliable PK data
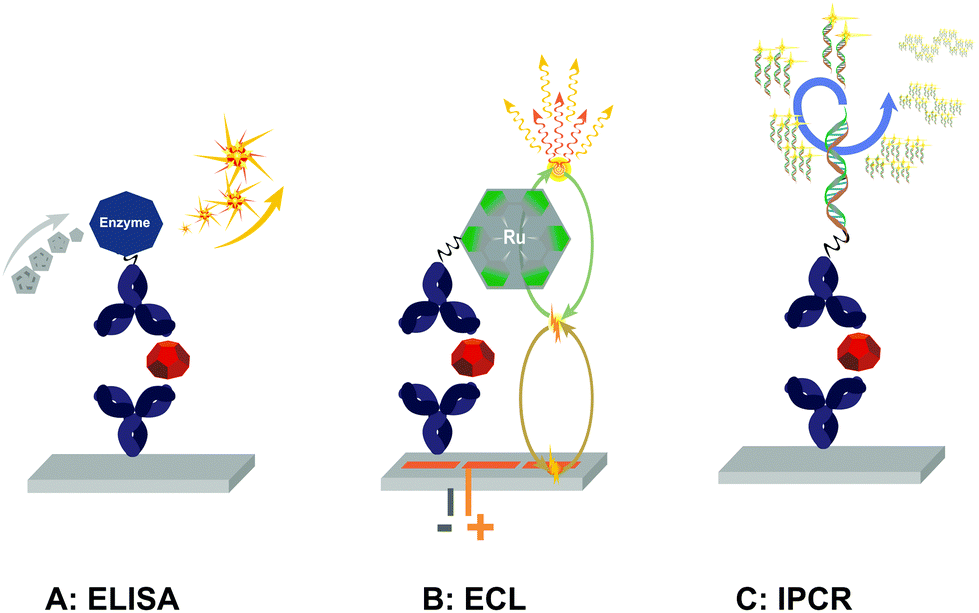



Highly Sensitive Ligand Binding Assays In Pre Clinical And Clinical Applications Immuno Pcr And Other Emerging Techniques Analyst Rsc Publishing Doi 10 1039 C5an002k



Highly Sensitive Ligand Binding Assays In Pre Clinical And Clinical Applications Immuno Pcr And Other Emerging Techniques Analyst Rsc Publishing Doi 10 1039 C5an002k
Before assay development and validation can be initiated, it is recommended to understand the physiochemical properties of the analyte Bioanalytical assay development is considerably informed by the details on protein binding and in vivo/in vitro metabolism along with essential aspects of prior work on the method Matrix background should be kept to a minimum in PK assays as it affects the assay sensitivity and may limit the quantitative assay range For ADA assays, it is recommended that both the upper and lower limits of the matrix response range be established as soon as validationDespite an increase in awareness about biomarker assay validation, there are still multiple instances in which laboratories are obtaining incorrect, inaccurate, or variable results because they are following PK guidance documents for assay validation, which potentially can present a




Amazon Com Fit For Purpose Biomarker Assay Development And Validation Dvd Movies Tv




An Assay Development Flowchart Download Scientific Diagram
Members of the AAPS Ligand Binding Assay Bioana tions of related regulations (11,12) in both bioanalytical and lytical Focus Group Biomarker Subcommittee subsequently Table I Comparison of Pharmacokinetic (PK) Drug, Biomarker, and Diagnostic Assays Biomarker assay for Biomarker assay PK assay drug development for diagnostic Intended useHowever, development of these therapeutics is challenging and only recently are promising clinical data emerging These challenges include ADC bioanalysis, such as quantifying in serum/plasma for PK studies and strategies for assessing immunogenicityPK studies in animals are critical for safely advancing the drug into clinical development We provide pharmacokinetics services during clinical trials by developing and validating bioanalytical methods in the human biological matrices post the FDA approval of your IND application




Biomarker Assay Development And Validation Failing Your Way To Success Celerion




Immunoassay Methods Assay Guidance Manual Ncbi Bookshelf
Development and Validation of a CellBased HighThroughput Screening Assay for TRPM2 Channel Modulators YUMEI SONG, 1BEN BUELOW,ANNELAURE PERRAUD,2 and ANDREW M SCHARENBERG1 TRPM2 is a member of the transient receptor potential melastatin (TRPM)–related ion channel family The activation of TRPM2 LigandBinding Assays covers essential topics related to ligandbinding assays, from pharmacokinetic studies, the development of LBAs, assay validation, statistical LBA aspects, and regulatory aspects, to software for LBAs and robotics and other emerging methodologies for LBAs Highlights include A general discussion of challenges and provenDevelopment, validation and implementation of PK and biomark er ligandbinding assays The goal of this ar ticle is t o discuss the fu ndament al key questions arou nd parallel ism assessme nts




0olvnr Yzrlsnm




Phases Of Drug Development Process Drug Discovery Process Northeast Biolab
Best Practices for the Development and Validation of qPCR and ddPCR Assays Posted CellBased Assays Learn how BioAgilytix provides the specialized large molecule insight and proven GLP / GMP Pharmacokinetics & Pharmacodynamics (PK/PD) See how our PK expertise complements our immunogenicity services by evaluating absorptionBreadth of Biologics' clinical trials with PK/ TK, ADA, Nab and Biomarker assays and sample analyses Versatile Performance and Project Management Excellence to adapt to a client's specific needs Clinical or preclinical, regulated or nonregulated, assay development, qualification or validation;Phases method development, prestudy validation, and instudy validation During method development, an assay concept is evaluated, which will be confirmed during the prestudy validation phase, and applied during the instudy validation phase To ensure that an assay can be used in the quantification




Potency Assays For Atmps Overcoming Challenges On The Path To Commercialization Insights From Our Labs To Yours




Assay Development Irbm
Studies and the assessment of pharmacokinetics (PK) and immunogenicity Although there are FDA, EMA guidelines, and whitepapers describing best practices for bioanalytical method development and validation 49, there is no clear guidance or recommendation to design and validate bioanalytical assays for biosimilars Premier Preclinical and Clinical Support for PK/PD Assay Development and Validation BioAgilytix understands that when it comes to PK assays, PD assessments, and DMPK assays, it is of the highest priority that the applied bioanalytical method is well characterized, fully validated, and documented in order to yield reliable resultsPBL has great expertise in the development of analytical methods and can quickly perform bioanalytical method development and validation of sensitive, accurate and robust PK assays PBL's chemists have experience working with plasma, serum, urine, CSF, and other tissues, and will optimize the extraction conditions to provide a reproducible




Specialty Lab Biomarker Assays Precision For Medicine




Immunogenicity Assay Development And Validation For Biological Therapy As Exemplified By Ustekinumab Mojtahed Poor 19 Clinical Amp Experimental Immunology Wiley Online Library
59 In an LBA validation, full validation should be conducted using samples diluted at a 60 factor of minimum required dilution (MRD), which has been determined in the course of 61 method development In a platebased LBA, assay should generally be performed in at 62 least duplicate (2 wells) per processed sample The sample concentration should beEnvigo were asked by a client to develop and validate a PK/TK assay for a monoclonal antibody used to treat cancer at levels as low as 10 ng/ml in cyno samples The company first transferred the assay from ELISA to the MSD platform before finally transferring it to Gyrolab™ system, where the development and validation work was completedAssay validation provides an assurance of reliability during normal use, and is sometime referred to as "the process of providing documented evidence that the method does what it is intended to do" Assay Validation Levels and Steps Assay Optimization (prevalidation) Assay optimization and prevalidation are experiments that determine
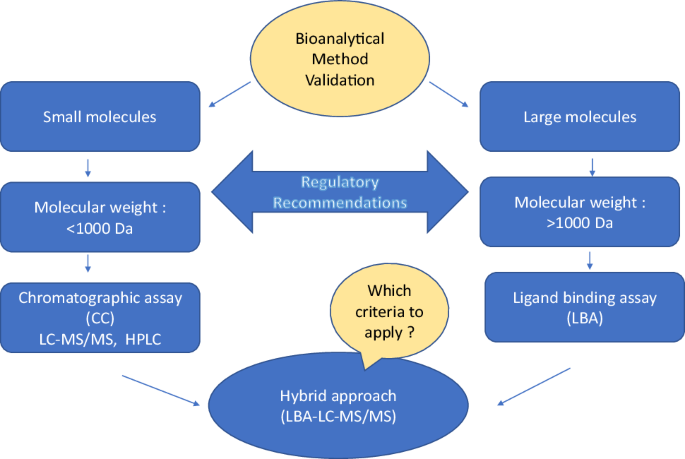



Review Of Recommendations For Bioanalytical Method Validation Chromatographic Assays And Ligand Binding Assays Springerlink



Www E B F Eu Wp Content Uploads 18 06 Fw1606 03 Marianne Scheel Fjording Ebf Pdf
Bioavailability (PK, ADME), toxicity In vivo efficacy Preclinical GLPTox Clinical Target Identification Target Validation Assay Development Lead Optimization Development HTS NonHTS Assay development A critical part of the "hit" discovery process "HITS" A "hit" is a compound whichBiomarker assay development and validation at Smithers follows the "FitforPurpose" concept described by Lee et al (06) Biomarker assay capabilities include ELISA (single analyte) Meso Scale Discovery (single and multiple analytes) Singulex (Ultrasensitive, single analytes) Quanterix Simoa HD1™ Biomarker Experience Method validation The Abconjugated payload assay is a hybrid assay which involves a ligand binding step and chromatographic analysis, therefore, a fitforpurpose approach to method validation was implemented based on BristolMyers Squibb Standard Operating Procedures (SOPs) for both chromatographic and ligand binding method validations




Assay Techniques And Test Development For Covid 19 Diagnosis Acs Central Science




Bioanalytical Assay Development And Validation Testing Services Northeast Biolab
Person X Someone told me assays need to be validated to start Phase 2 studies • Me No, unless there is a scientific reason, submission of assay validation isn't required until the BLA is submitted However, you need to be using qualified assays from the beginning If the assays you're using during later phases of clinical developmentGetting a firm grasp of the PK and TK properties of your drug is essential for preclinical and clinical development and selecting a dose Eurofins' industryleading experts have experience in the development and validation of PK/TK assays and offer a wide range of TK and PK services for large molecules and peptidesApplication of PK in optimizing drug therapy and evaluating bioavailability was truly made possible by the skills of the analytical chemists who pioneered the development of HPLC in the 1970s During the 1980s, HPLCUV based assays routinely provided the plasma concentration data that were used to define drug exposure in
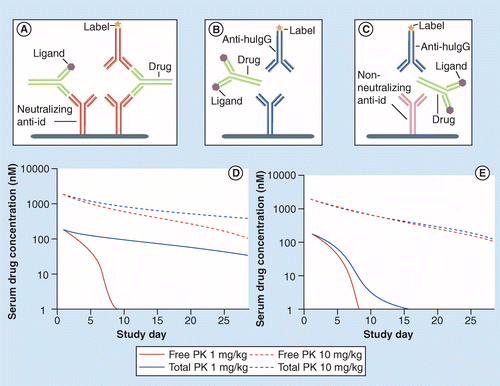



Pk Pd Modeling Of Protein Drugs Implications In Assay Development Bioanalysis
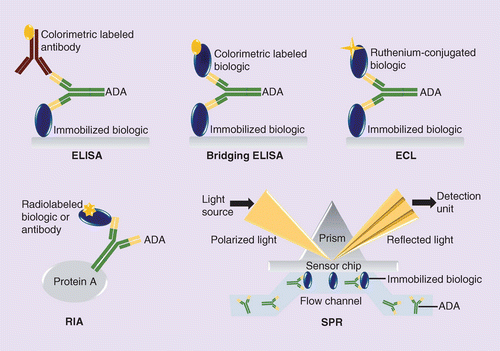



Comparative Immunogenicity Assessment Of Biosimilars Future Oncology
The following sections discuss the development, validation, and instudy use of bioanalytical methods and how best to document validation methods and results Refer to the Glossary for theA high performance generic PK assay recognizing human monoclonal antibody eliminates the need for multiple rounds of assay/reagent development and validation to support experimental activities using less sample volumes for a variety of studies early in development179 the bioanalytical data in support of the development and market approval of both chemical and 180 biological drugs 181 The objective of the validation of a bioanalytical assay is to demonstrate that it is suitable for its 1 intended purpose Changes from the recommendations in this guideline may be acceptable if




Immunogenicity Assessment Of Biotherapeutic Products An Overview Of Assays And Their Utility Sciencedirect




Table I From Fit For Purpose Method Development And Validation For Successful Biomarker Measurement Semantic Scholar
We custom design our support to In recent years, there has been growing appreciation that biomarker assay development and validation must be considered in their own context and not incongruously fitted into concepts/approaches developed to evaluate PK assay performancePharmacokinetics (PK) studies are of great importance in creating new therapeutic drugs, understanding both the beneficial and harmful effects of drugs, and guiding drug development experiments and trials In PK assays, the applied assay method should be well characterized and fully validated, in order to yield reliable results



Development And Validation Of Uplc Ms Ms Method For Studying The Pharmacokinetic Interaction Of Dasabuvir And Tamoxifen 4 Hydroxytamoxifen In Wistar Rats Scientific Reports



Flow Cytometry Assay Validation As A Biomarker Versus In A Drug Development Setting Cellcarta
The recommendations for assay development 24 and validation provided in this document apply to assays for detection of antidrug antibody(ies) 25 (ADA) 4And Quanterix SIMOA ® for ultrasensitive detectionPK Assays We offer assay development, validation, and implementation using ELISAs and MesoScale Discovery (MSD) assays for biologics;




Pk Tk Gyrolab Automated Immunoassays Gyros Protein Technologies




Pharmacokinetic Pk Assays Pk Pd Analysis Dmpk Assay




Parallelism Considerations For The Development Validation And Implementation Of Pk And Biomarker Ligand Binding Assays Bioanalysis Zone




Pdf Parallelism Considerations For The Development Validation And Implementation Of Pk And Biomarker Ligand Binding Assays




Iq Consortium Perspective Complementary Lba And Lc Ms In Protein Therapeutics Bioanalysis And Biotransformation Assessment Bioanalysis



2



Onlinelibrary Wiley Com Doi Pdf 10 1002 Path 4262



E B F Eu Wp Content Uploads 18 06 Fw1606 05 Liz Hickford Ucb Biopharma Pdf



3



Ligand Binding Assays Development Validation And Implementation In The Drug Development Arena Edition 1 By Masood N Khan Hardcover Barnes Noble



Development And Validation Of Pharmacokinetic Pk And Immunogenicity Assays Supporting Demonstration Of Biosimilarity



Full Article Bioanalytical Strategy Used In Development Of Pharmacokinetic Pk Methods That Support Biosimilar Programs




Biomarker Assay Development And Validation Failing Your Way To Success Celerion
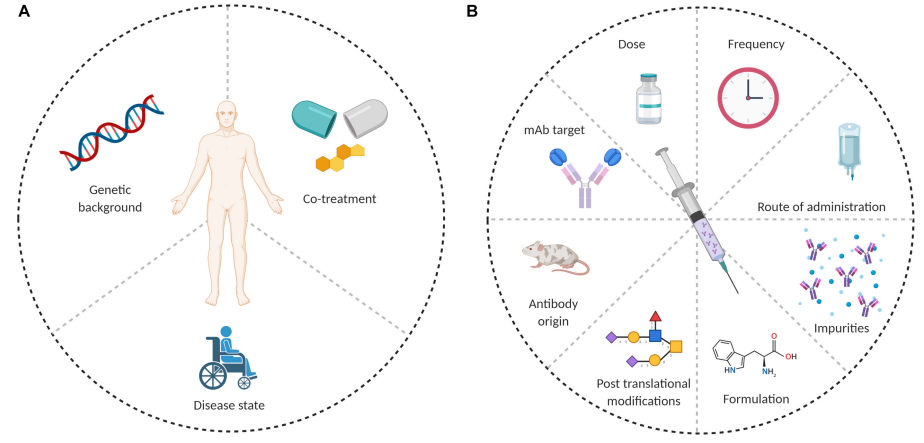



Anti Drug Antibodies Development Creative Biolabs



3




Parallel Anticancer Drug Development And Molecular Stratification To Qualify Predictive Biomarkers Dealing With Obstacles Hindering Progress Cancer Discovery




Integrated Biomarker Strategy Biomarker Development Validate And Qualify Or Is It The Other Way Around




Pharmacokinetic Studies In Children Recommendations For Practice And Research Archives Of Disease In Childhood




Nonclinical Development Basics The Right Nonclinical Strategy And Study Designs Camargo



3




Cell And Gene Therapy Absorption Systems
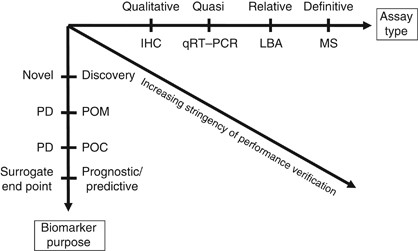



Fit For Purpose Biomarker Method Validation For Application In Clinical Trials Of Anticancer Drugs British Journal Of Cancer
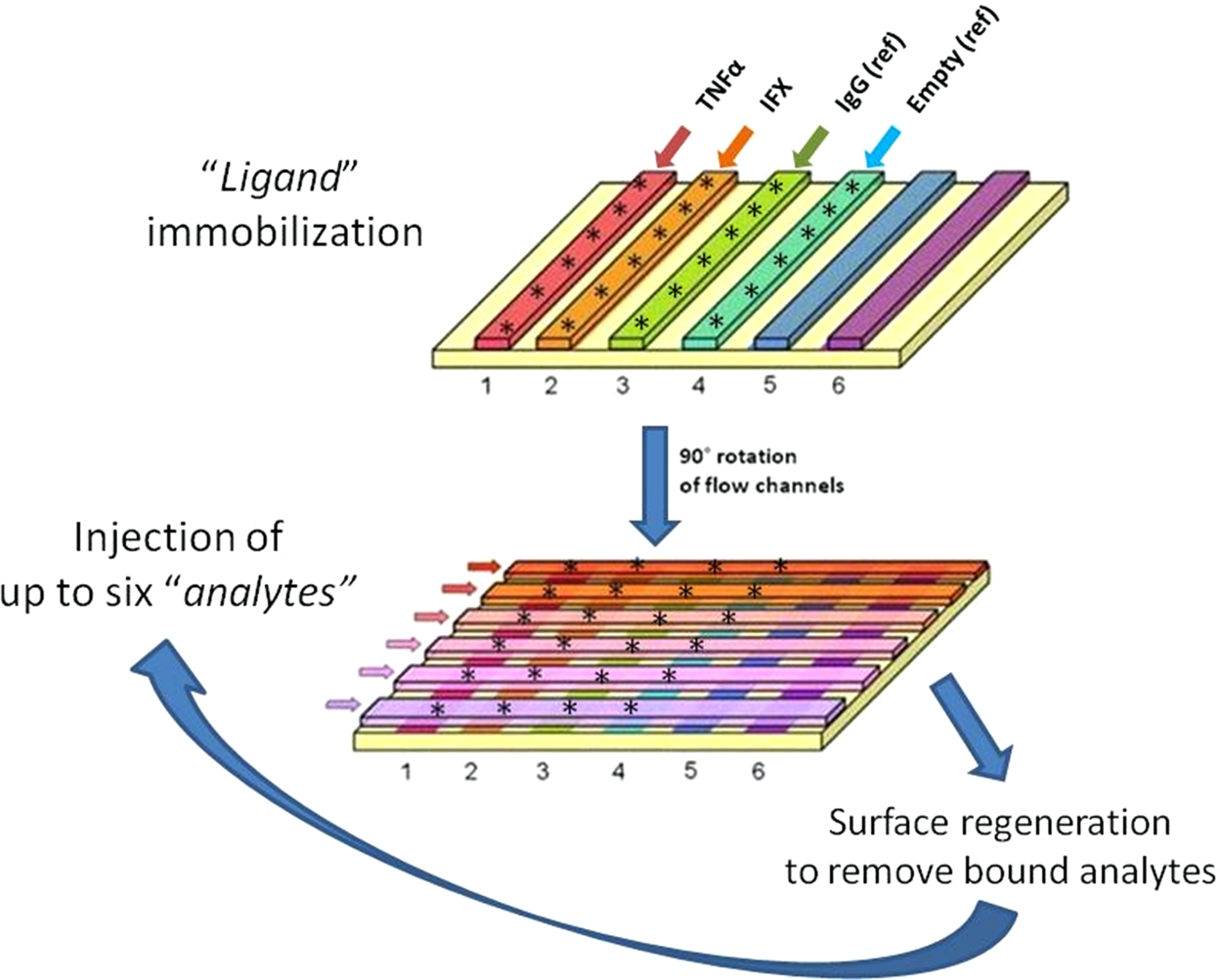



A Surface Plasmon Resonance Based Assay To Measure Serum Concentrations Of Therapeutic Antibodies And Anti Drug Antibodies Scientific Reports




Biomarker Assay Translation From Discovery To Clinical Studies In Cancer Drug Development Quantification Of Emerging Protein Biomarkers Sciencedirect




Biosimilar Development Biosimilar Biological Products Development Applications




Uzivatel Microconstants Na Twitteru Microconstants Offers Customized Method Development And Validation For Your Bioanalytical Needs Visit Our Website To Learn More Around Small And Large Molecule Method Development And Validation T Co




Development And Qualification Of A Characterisation Panel To Assess The Biological Activity Of Golimumab Simponi Eurofins Scientific




Nab Tab Ada Assays Precision For Medicine




Assay Guidance Manual Ncbi Bookshelf
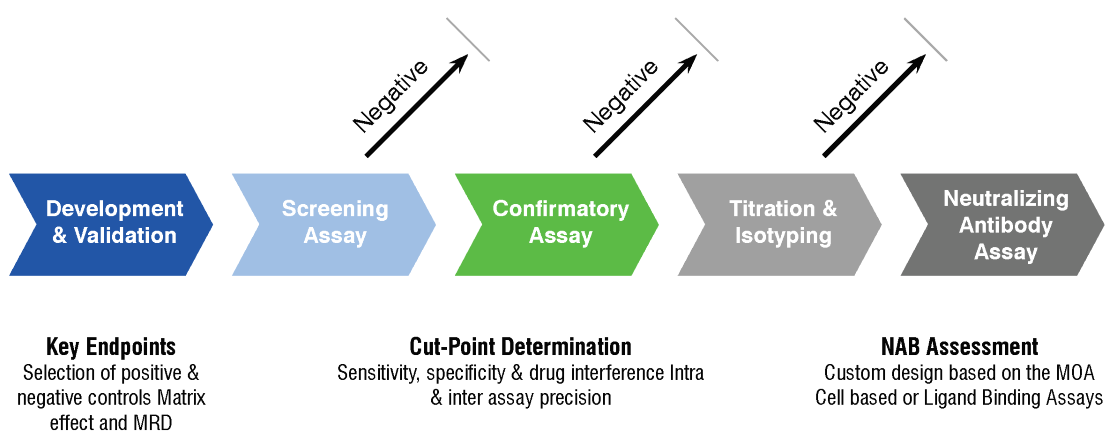



Immunogenicity Testing Charles River
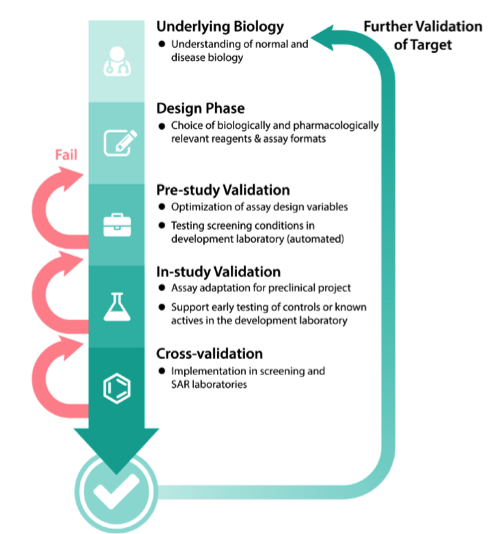



The Blog How To Accelerate Assay Development And Reduce Cycle Times



2




Preclinical Bioanalysis Wuxi Apptec Lab Testing Division
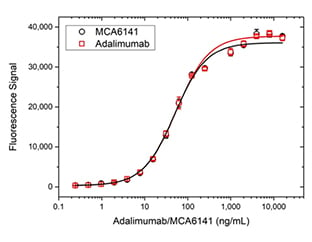



Biosimilar Antibodies For Research Use Bio Rad




Pk Pd Modeling Of Protein Drugs Implications In Assay Development Bioanalysis
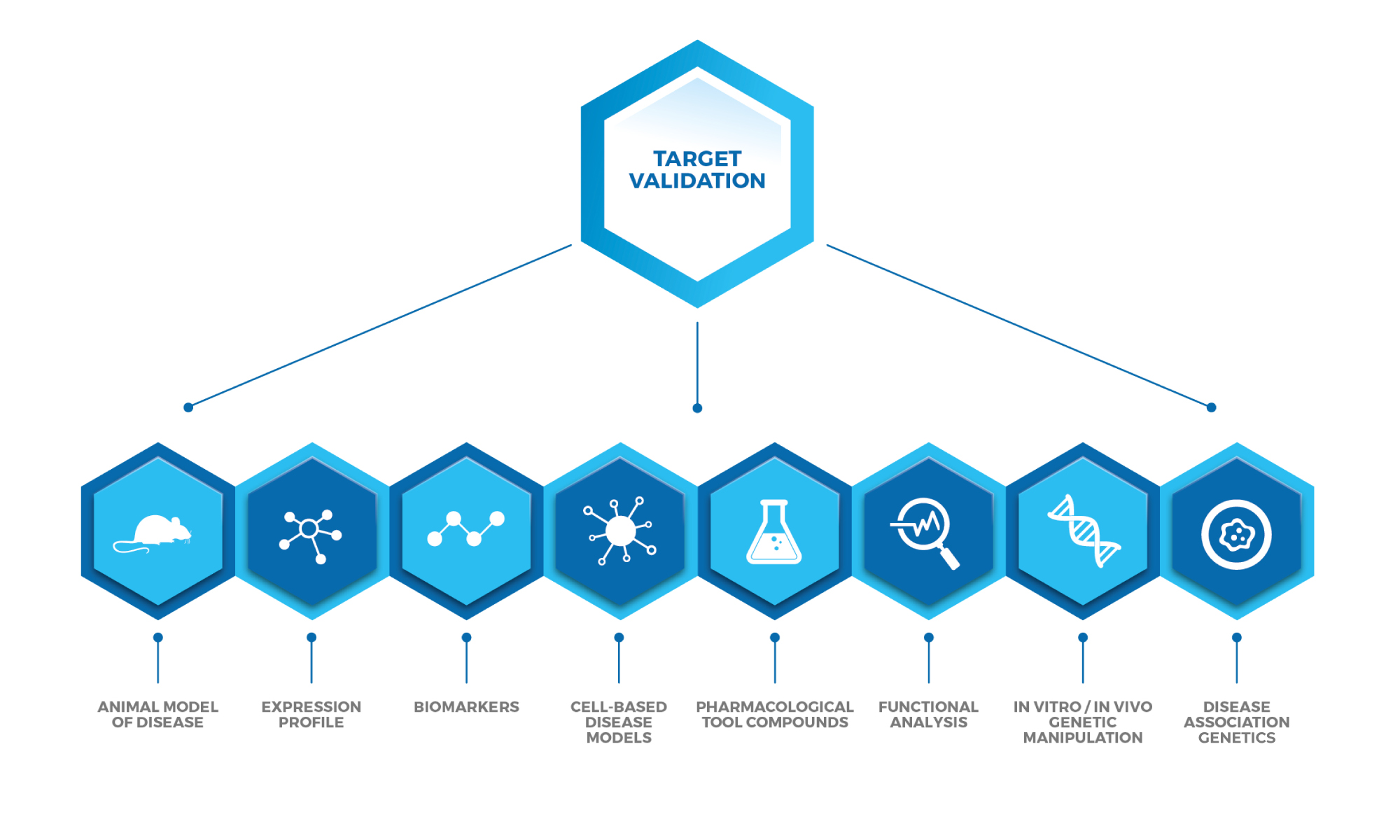



Target Validation In Drug Discovery Sygnature Discovery
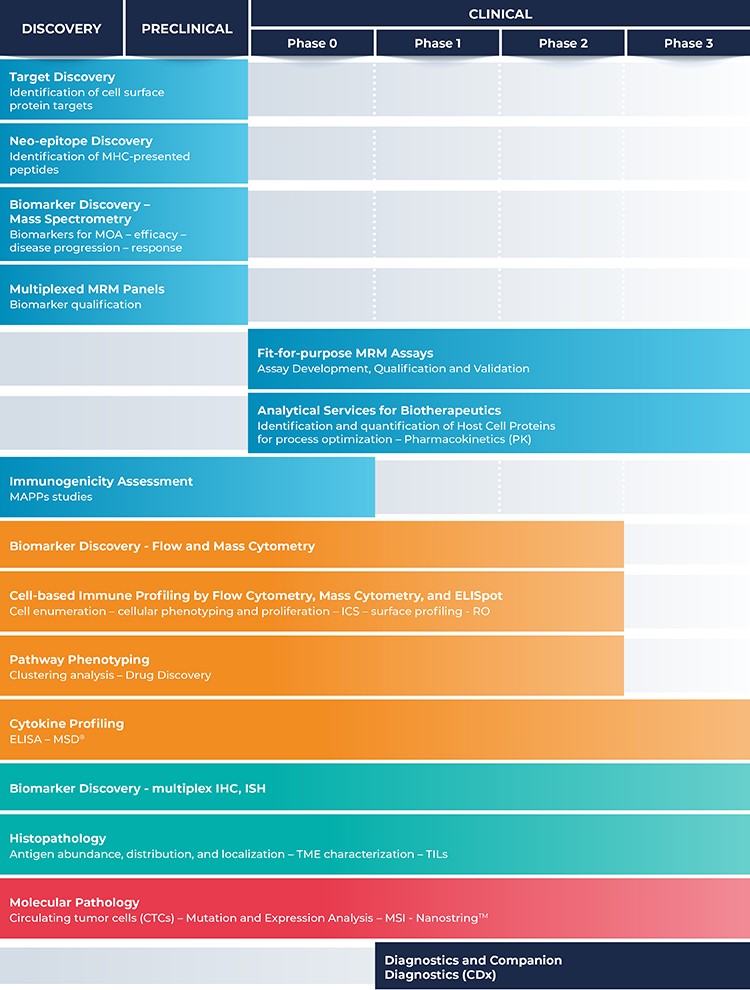



Discovery Phase Cellcarta




Bioanalytical Method Development And Validation Critical Concepts And Strategies Sciencedirect



Assay Development In Vitro And In Vivo Taiwantrade Com




Research Phases Nms Labs




Using Scientist For Contract Pharmaceutical Research Outsourcing Scientist Com



Biomarkers Clinical Trials A Quality Driven Integrated Approach Pharmavoice
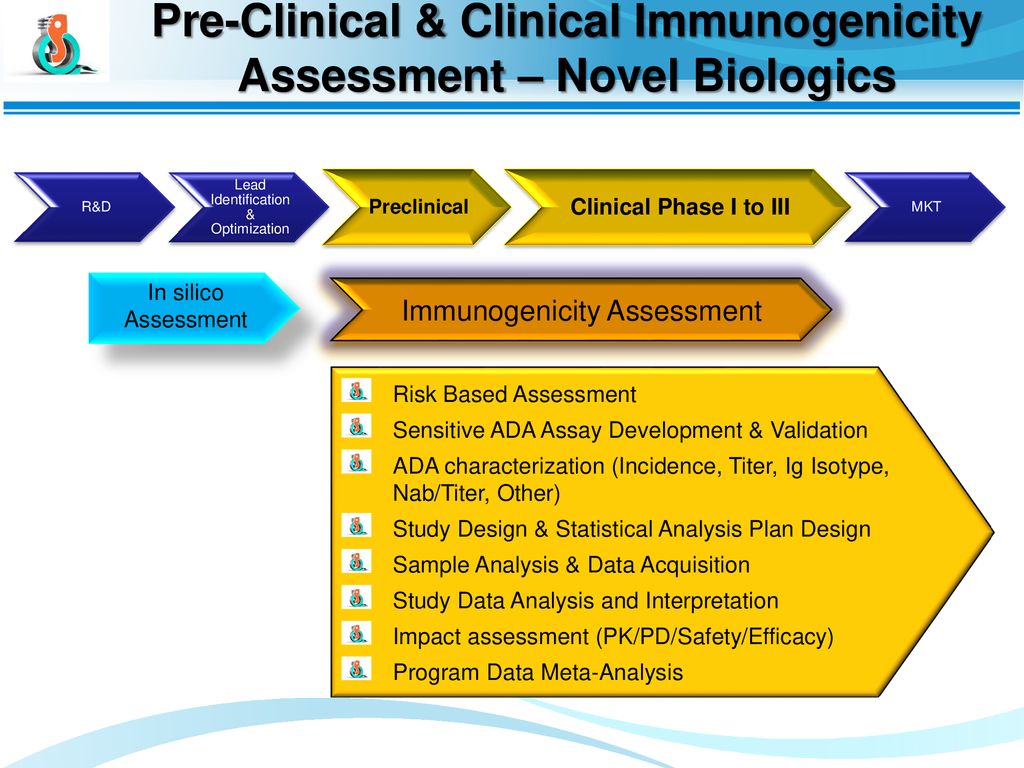



Evaluation Of Immunogenicity In Biotherapeutics Ppt Download



1
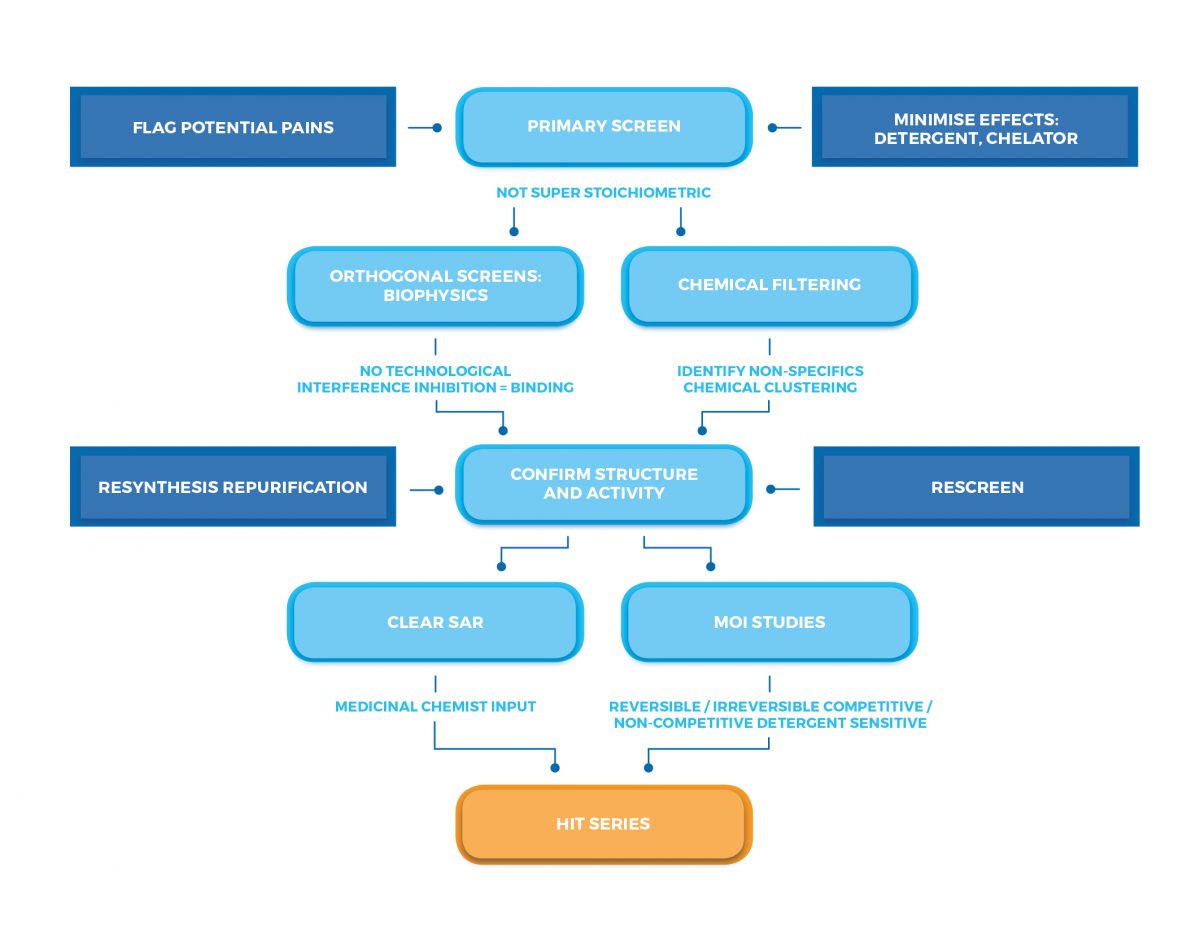



Cascade Screening Development Services Sygnature Discovery



Cdn Ymaws Com Www Casss Org Resource Resmgr Cmc No Am Jan Spkr Slds 18 Cmcj Cieslakjacek Pdf



Www Uab Edu Medicine Adda Images Padmalayamassaydevelopment Pdf



Assay Validation Biomarker Assay Validations A Time For Change



Pdf Parallelism Considerations For The Development Validation And Implementation Of Pk And Biomarker Ligand Binding Assays




Wieslab Help Accelerate Drug Development Programs Wieslab
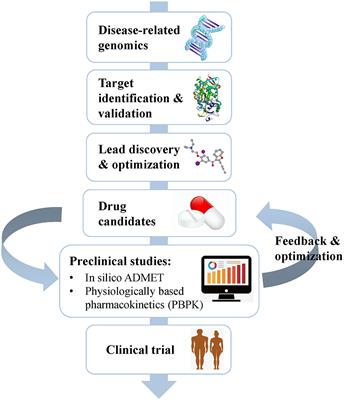



Frontiers Computational Approaches In Preclinical Studies On Drug Discovery And Development Chemistry



Target Validation Biochemical And Cellular Assay Development




Drug Discovery And Development Flashcards Quizlet



Highly Sensitive Ligand Binding Assays In Pre Clinical And Clinical Applications Immuno Pcr And Other Emerging Techniques Analyst Rsc Publishing Doi 10 1039 C5an002k




Qpcr And Qrt Pcr Analysis Regulatory Points To Consider When Conducting Biodistribution And Vector Shedding Studies Molecular Therapy Methods Clinical Development
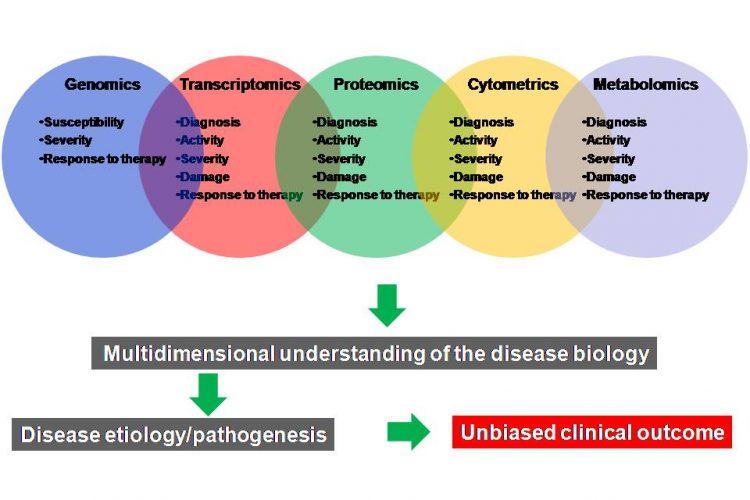



Biomarkers In Drug Discovery And Development




Systematic Verification Of Bioanalytical Similarity Between A Biosimilar And A Reference Biotherapeutic Committee Recommendations For The Development And Validation Of A Single Ligand Binding Assay To Support Pharmacokinetic Assessments Semantic Scholar




Pdf Biomarker Assay Translation From Discovery To Clinical Studies In Cancer Drug Development Quantification Of Emerging Protein Biomarkers Semantic Scholar



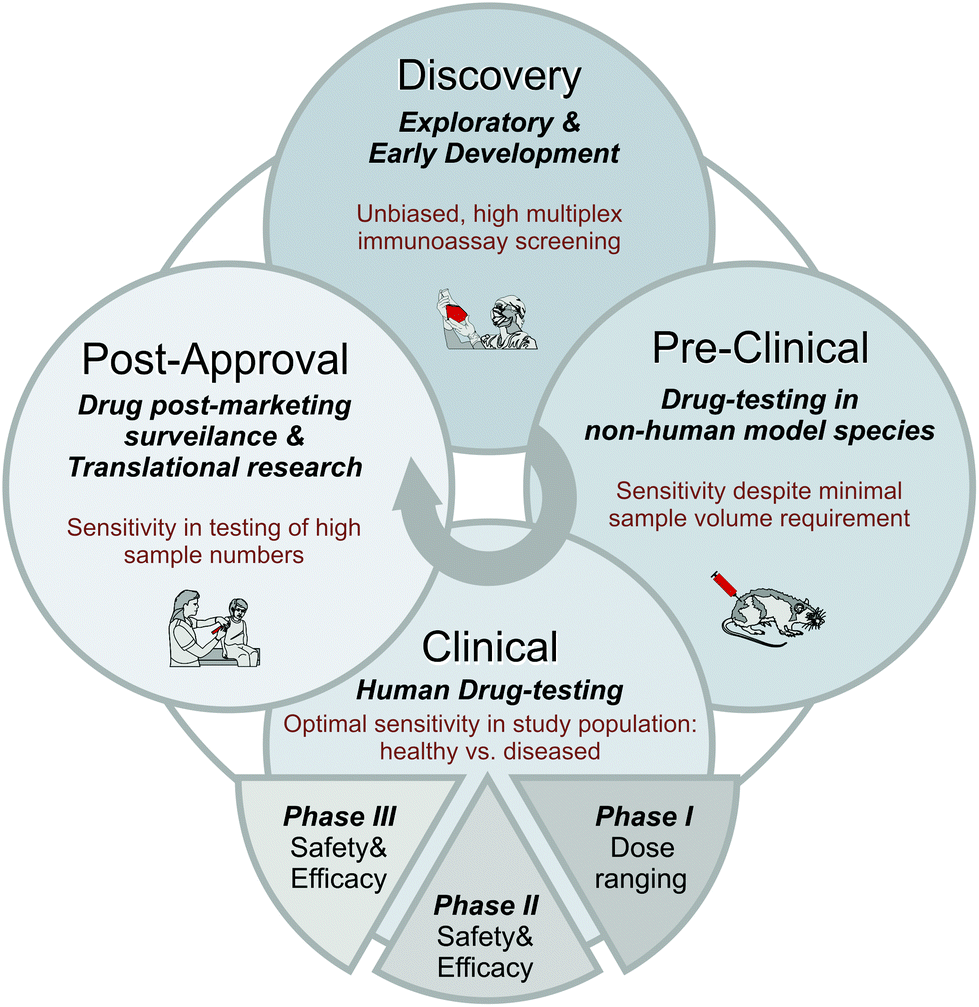
Bioanalytical Solutions Syneos Health




An Assay Development Flowchart Download Scientific Diagram
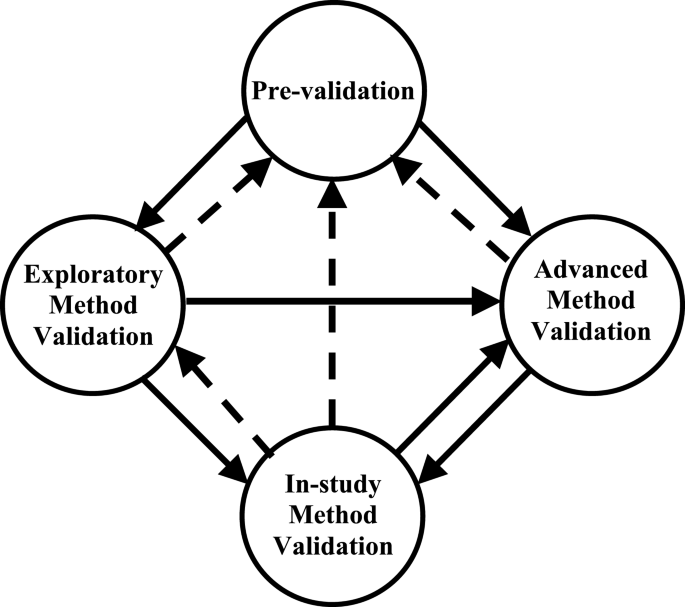



Fit For Purpose Method Development And Validation For Successful Biomarker Measurement Springerlink




Qpcr And Qrt Pcr Analysis Regulatory Points To Consider When Conducting Biodistribution And Vector Shedding Studies Molecular Therapy Methods Clinical Development




4 Do You Follow A One Assay Approach Or Two Assay Approach For Your Pk Bioanalytical Assays What Are The Pros And Cons Bioanalysis Zone
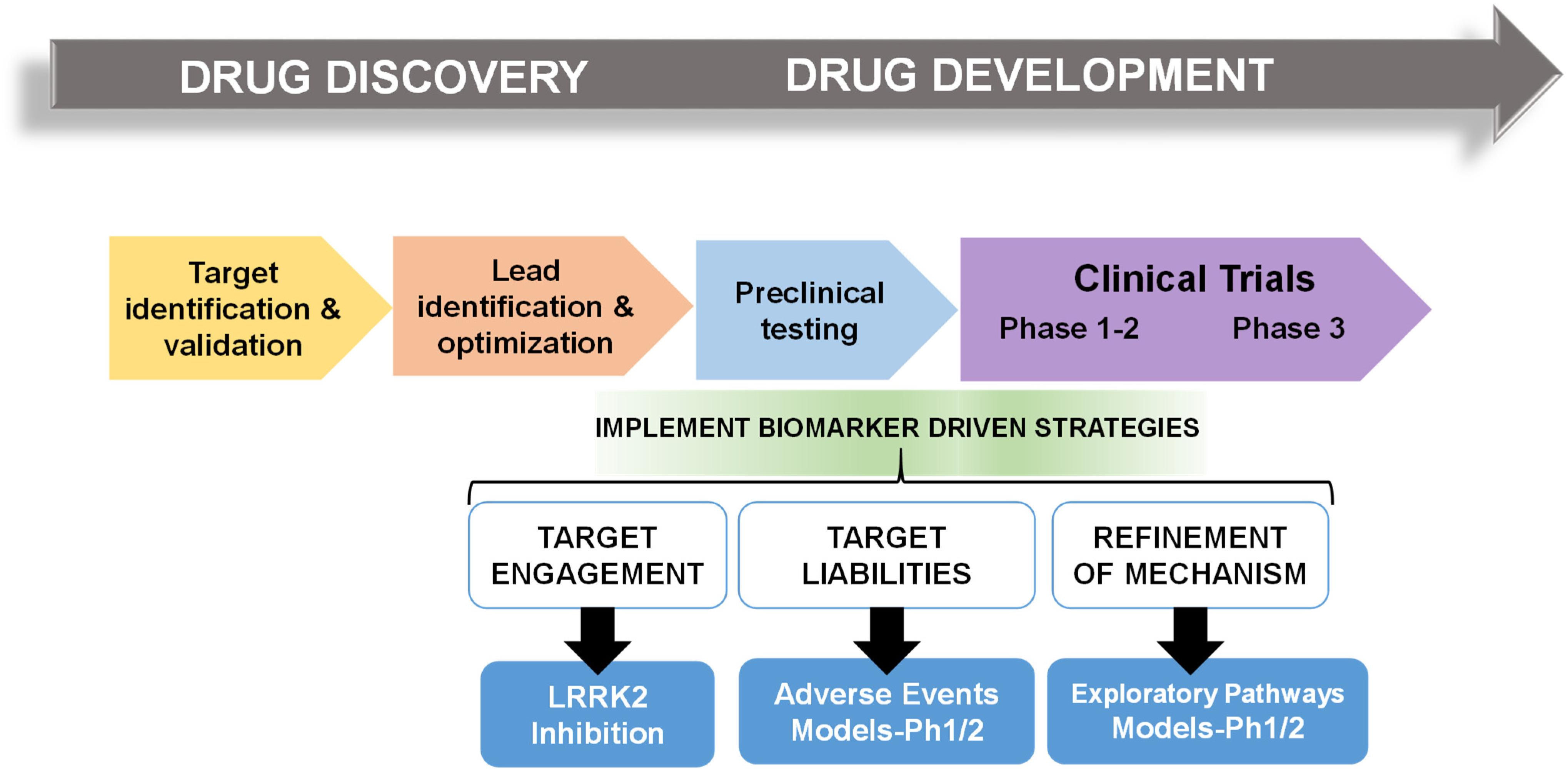



Frontiers Pharmacodynamic Biomarkers For Emerging Lrrk2 Therapeutics Neuroscience



Www Parexel Com Files 6213 9015 4087 Validated Assays July 13 Print Ready V2 Pdf




Drug Discovery Basic Research Drug Development
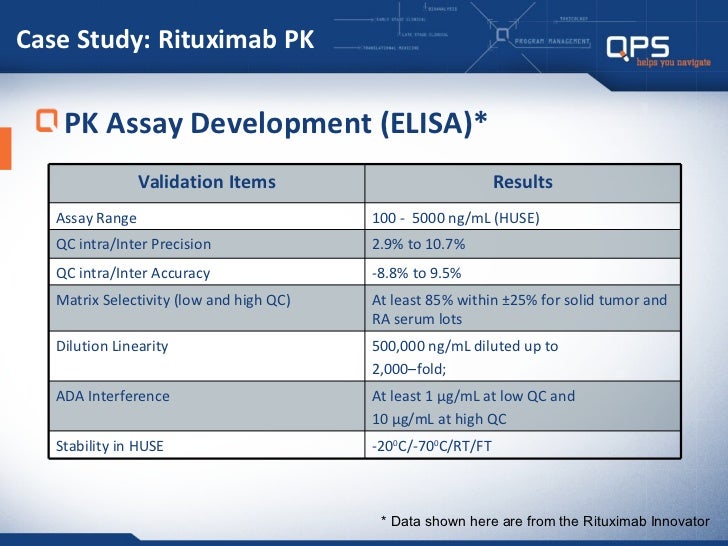



Qps Biosimilar Bioanalytical Approaches




Preclinical Development Wikipedia
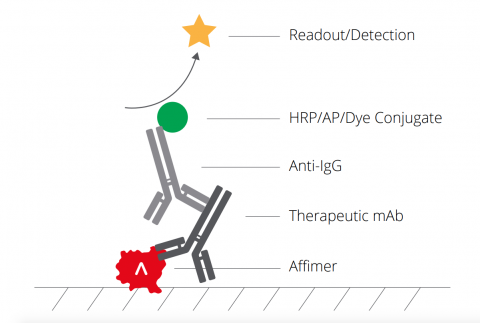



Improving Drug Development Processes With Antibody Pk Assays Avacta Life Sciences Limited




Integrated Lab Performance And Biopharma Solutions Eurofins Scientific




Development And Validation Of A Methotrexate Adherence Assay Annals Of The Rheumatic Diseases




New Fda Guidance On Developing Validating Ada Assays For Ada Antibody Detection




Mercodia Bioanalytical Services




Pk Tk Gyrolab Automated Immunoassays Gyros Protein Technologies



Immunogenicity Testing Regulatory Updates For Immunogenicity Assessment Of Therapeutic Proteins
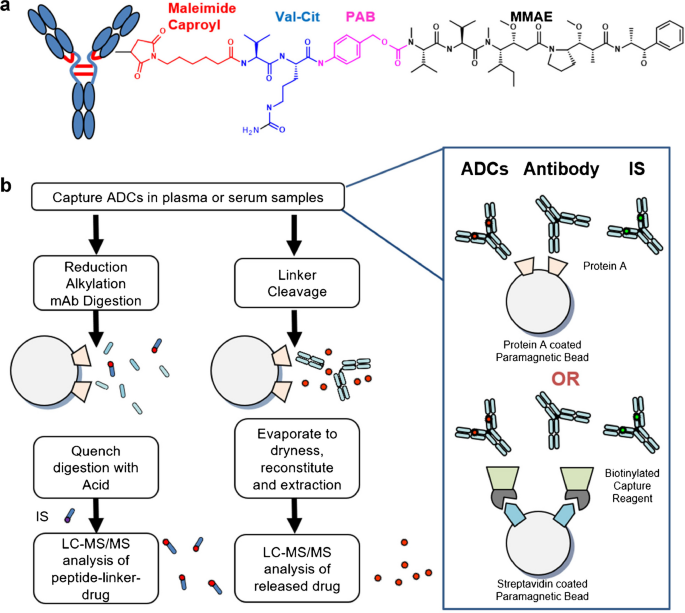



Method Development Of A Novel Pk Assay For Antibody Conjugated Drug Measurement Of Adcs Using Peptide Linker Drug Analyte Springerlink




Parallelism Considerations For The Development Validation And Implementation Of Pk And Biomarker Ligand Binding Assays Bioanalysis




Amazon Com Fit For Purpose Biomarker Assay Development And Validation Dvd Movies Tv



0 件のコメント:
コメントを投稿